How are drugs designed and developed?
Image credit: Shutterstock

Drug development is the process that takes us from an idea in the lab through extensive testing to a final product that can be used to treat people with specific conditions.
- Drugs are molecules, chemicals or biological substances that can cause a change in an organism’s physiology.
- Producing a new drug is an expensive process that is subject to extensive regulation and can take many years to ensure the new medicine is safe and effective.
What is a drug?
- Drugs are chemical or biological substances that have some kind physiological or chemical effect on the body.
- Their effects are intended to be beneficial but can cause harmful side effects in some people.
- They may be single compounds or a mixture of different compounds.
- All drugs interact with specific ‘targets’ in the body, with the aim of modifying their activity and often resulting in a therapeutic effect – such as pain relief or to treat conditions such as cancer.
- Drug targets are usually proteins but are in some cases small regions of DNA or RNA.
- Often, drugs work by stimulating or blocking the activity of their targets.
How is a drug developed?
- The development of a new therapeutic drug is a complex, lengthy and expensive process.
- It can take 10-15 years and over £500 million to develop a drug from an initial concept, test its safety and effectiveness in humans and then get it into the hospital market.
- Some parts of the drug development process are becoming quicker, thanks to the development of CRISPR-Cas9 and other precise gene editing technologies.
- This is because drug targets can be identified more quickly, and new drugs can be tested more easily in model organisms that are more realistic.
- During the Covid-19 pandemic, we needed new vaccines much more quickly than the traditional process – and new vaccines were developed in months rather than years. This is partly thanks to unprecedented collaboration between scientists, doctors, manufacturers and regulatory agencies to overcome challenges with funding, recruiting volunteers and negotiating with manufacturers.
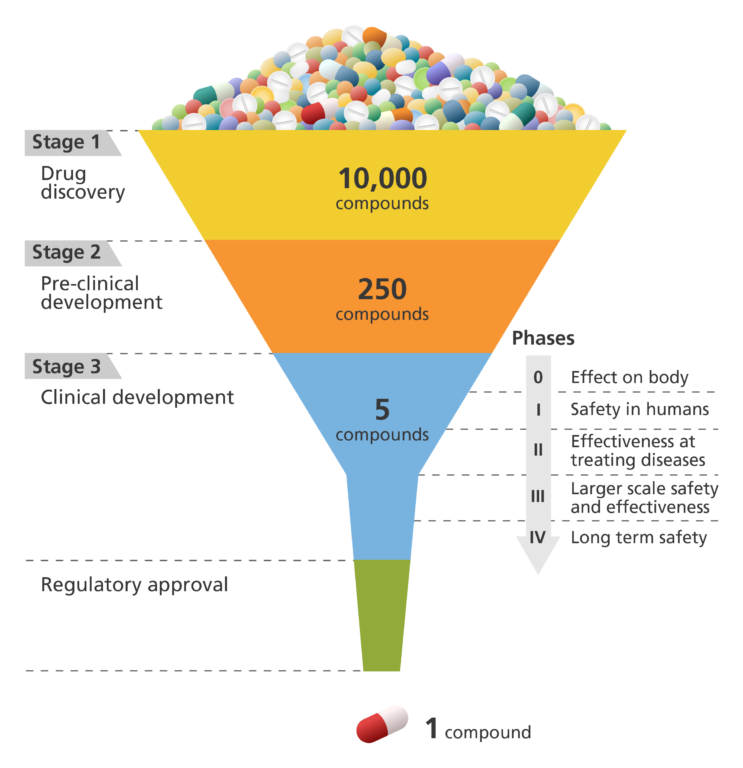
What are the stages of drug development?
Stage 1: Drug discovery
- The first stage of the drug development process is drug discovery.
- In the past, some drugs have been discovered by accident – such as penicillin.
- Today, more systematic approaches are used. This can include:
- high-throughput screening: which allows scientists to test thousands of potential targets with thousands of diverse chemical compounds to identify a new drug-target combination.
- rational drug design: which involves designing and synthesising compounds based on the known structure of a specific target molecule. Scientists often use artificial intelligence and machine learning to take this approach.
- High-throughput screening can identify hundreds of potential molecules that could form the basis of the drug. However, many are eliminated at the first round of testing. This is because they are found not to be safe or effective when tested in cultured cells or model organisms.
- Rational drug design develops fewer compounds compared to high-throughput screening. However, these compounds are very specific to the target and use computer-based modelling to achieve this specificity.
Stage 2: Pre-clinical development
- Pre-clinical testing is used to determine how best to develop the drug for its intended use.
- This step aims to establish how drugs are absorbed and distributed in the body, and how they are broken down and removed from the body.
- If appropriate, promising drugs may be modified to improve their properties in subtle ways, in a process called lead optimisation.
- The results of pre-clinical testing are also used to determine how to best formulate the drug for its intended clinical use – for example whether it would be most effective as a cream, a pill, an injection or a spray.
- The pre-clinical studies aim to whittle hundreds of compounds down to just a few useful candidate drugs.
- These few drugs will then be submitted to the appropriate regulatory authorities and, if accepted, the compound can be taken on to clinical development.
- This step is sometimes called ‘translation’ – translating a promising molecule from experiments in the lab into a real drug.
Stage 3: Clinical development (or clinical trials)
- Clinical development is divided into Phases 0, I, II, III and IV. These are also known as clinical trials.
- Clinical development involves testing the drug on human volunteers to provide more information about its safety and effectiveness.
- By the end of the clinical development phase, the safest, most effective investigational new drugs will be submitted as a new drug application. In the UK, this is known as a market authorisation application.
Stage 4: Marketing and regulatory approval
- After a drug has been approved by the appropriate regulatory bodies, the pharmaceutical companies who were involved in the drug’s development have a short period where only they have the rights to market the drug.
- This is called the exclusivity period and enables companies to regain the massive investment required to develop and launch the new drug. After this period, other companies can market the same drug.
- After full approval, drug companies must continue to test their drug and monitor feedback from healthcare professionals to ensure the safety and effectiveness of the drug.
- After launching a drug, new side effects or risk factors may be identified that had not been previously recorded. This is Phase IV of clinical development and is part of the continued monitoring of the effectiveness of the drug in their target patients.