How do we use CRISPR gene editing to study diseases?
Image credit: Shutterstock
CRISPR-Cas9 is a genome editing tool used to alter specific sequences of DNA in a cell. It has many uses both in research and in helping us understand disease.
- Many fantasies of gene editing have been made reality with the development of the gene editing tool known as CRISPR-Cas9. Whilst it might sound like a sci-fi gadget, it is making a very real impact on the study of disease.
What is CRISPR-cas9?
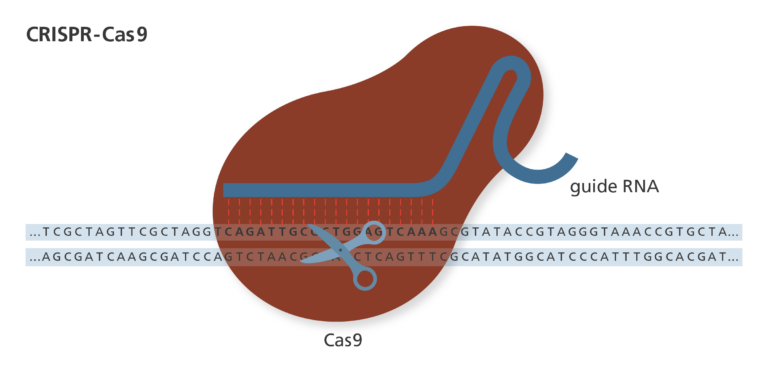
- Gene editing is used by scientists to alter specific sequences of DNA within a cell. To do this, the CRISPR-Cas9 system needs an enzyme to edit DNA sequences, and something to guide the enzyme to the correct place.
- The enzyme which edits DNA is called Cas9, and it is often described as a pair of genetic scissors. This is because it can cut DNA strands, allowing things to be added or removed from the ends of the cut strands before they are re-attached.
- In order to make sure that the Cas9 enzyme cuts in the right position, it is guided by the fittingly named guide RNA. This RNA molecule is designed to bind to a specific sequence of DNA bases and makes sure that Cas9 only causes breaks where they are wanted.
How is CRISPR used in the lab?
- When Cas9 makes a cut in a DNA molecule, it leaves exposed ends where bases (the building blocks of DNA) can be removed or added. This means a scientist can change the DNA sequence as they choose.
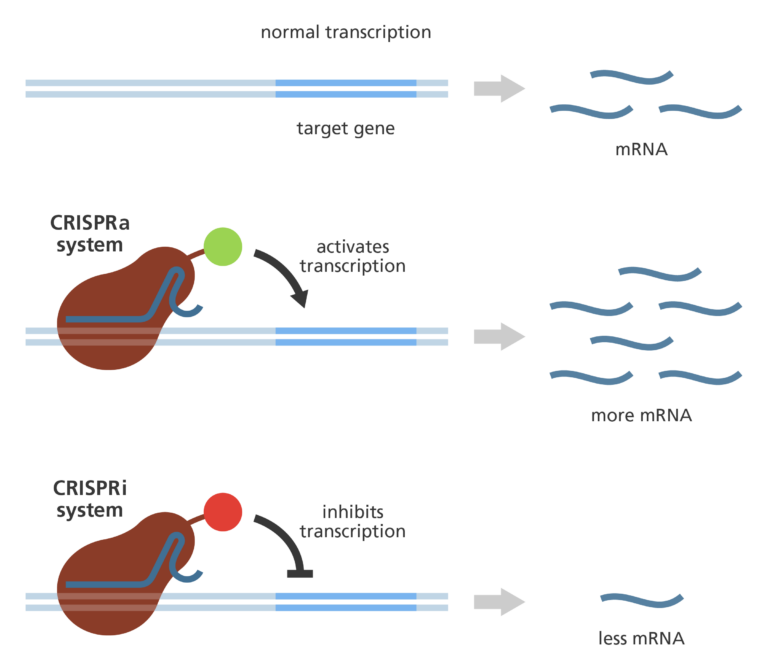
- The Cas9 enzyme can be adapted for different functions, which allow scientists to do even more things to the specific DNA site. One very useful adaptation involves removing the cutting function from Cas9 and replacing it with a different type of enzyme.
- Most commonly, the new enzymes used to replace Cas9 are designed to activate (increase), or inhibit (reduce) transcription of a specific gene. These methods are known as CRISPRa, meaning CRISPR-activation, or CRISPRi, meaning CRISPR-interference.
What can we learn from CRISPR about diseases?
- Many diseases have a genetic element – but we don’t always know much about how the genes are involved or which genes are behaving abnormally.
- When genes act abnormally, they might make a faulty protein, or they might produce too much or too little of a protein. This can lead to a metabolic disease such as cystic fibrosis or sickle cell anaemia. If the protein is involved in the control of the cell cycle or apoptosis, abnormal genes may lead to cancers.
- Often CRISPR-Cas9 is used in the lab to investigate unknown functions of genes. Disrupting the sequence or transcription of a gene allows scientists to ask many questions about how that gene works, including:
- What happens when a gene is deleted (knocked-out)? How does a cell or model organism behave differently when a particular gene is missing?
- What is the effect of small specific changes to a sequence? These changes, known as mutations, often occur naturally. By recreating them in the lab we see if they have any impact on processes within a cell, such as disrupting protein function.
- If transcription of a gene is turned up or down, does this change the characteristics of the cell or organism?
- When studying genetic diseases, DNA-sequencing allows scientists to look at different mutations occurring across individuals and populations. This information allows us to identify mutations found more frequently in people with specific diseases, suggesting that the mutation increases the chance of the disease occurring.
- To test the link between mutations and specific diseases further, scientists investigate whether producing a specific mutation causes a cell to switch from healthy to diseased. This technique is becoming increasingly useful in cancer research.
- The same approach is used to see if a specific genetic change causes other interesting biological effects - for example, does changing a sequence in a bacterium allow it to become resistant to drug treatment? This might help us tackle antibiotic resistance in future.
CRISPR in the future
- There is huge potential for CRISPR to be used as a treatment for disease, not just a tool for studying it.
- One example is in an immune cell therapy for cancer patients. Doctors remove a type of immune cell called T cells from a patient, and modify their DNA to train them to kill cancer cells. The modified T cells are returned to the patients’ bloodstream. CRISPR technology is being investigated as a better way of editing these cells, compared to previous gene editing methods.
- In future CRISPR could be used in embryos to change mutations known to increase the risk of disease.
- There is much debate about the ethical implications of editing human cells, especially when these changes are made to a person’s germline (reproductive) cells and could be inherited by future generations.
- By editing the DNA of crop plants, CRISPR will help create crops which produce a lot more food, or become resistant to drought. These increased yields will help overcome shortages of food, agricultural land, and water.
Article written by Olivia Edwards, PhD Researcher at the Wellcome Sanger Institute.