How inbreeding mice led to lifesaving treatments

Humans have been breeding animals for millennia to bring out desirable characteristics. In research, this has led to the mouse model – a valuable tool for studying genetics and disease.
Key terms
Gene
A section of DNA within a genome that carries a specific set of information - often the information needed to make a protein.
Mutation
A change in the DNA sequence, which may have positive, negative or neutral effects on the organism.
Model organism
A species that has been widely studied in biology, usually because it is easy to maintain and breed in a laboratory setting and has particular experimental advantages.
Cancer
A common genetic disease caused by mutations in DNA, and characterised by uncontrolled cell growth.
For thousands of years, humans have bred animals and plants to bring out characteristics society found useful. This selective breeding in agriculture has given us different types of cereals, fruit and vegetables, as well as livestock, like cows bred for milk or meat production. In research, this has given us model organisms – non-human species, such as the mouse, fruit fly and nematode worm – that can help us understand specific areas of biology.
Breeding siblings or close relatives together over several generations eventually results in genetically identical offspring that are clones of their parents. Known as inbreeding, pure breeding or ‘breeding true’, this practice has developed valuable tools to enable scientists to study basic biology, health and disease – leading to new, important, effective treatments.
Introducing C57Black6 – a strain of mouse that would become very important
In the early 1900s, ex-teacher Abbie Lathrop bred mice with interesting features and characteristics, initially to sell as pets. ‘Waltzing’ mice had the strange behaviour of rapidly and constantly whirling around in circles, while ‘fancy’ mice were bred for their appearance and temperament.
Abbie kept careful records of her mice, as the numbers on her farm grew to around 11,000. For example, she noticed that some mouse families developed breast cancer more than others. When she removed the ovaries from these mice, their chance of developing breast cancer dropped – something we now know can also help reduce the likelihood of cancer in people with high risk of the disease.
Other scientists began working with Abbie and her mice, which were valuable for medical and genetic research due to their similarity with humans and the ability to breed them in a quick, controlled way.
For example, Clarence Cook Little began using the mice in the 1920s to study genetics and cancer. He inbred the mice over many generations – and because extreme inbreeding can lead to negative characteristics, he excluded families which became ill or were more likely to develop genetic diseases and started again if the colony died.
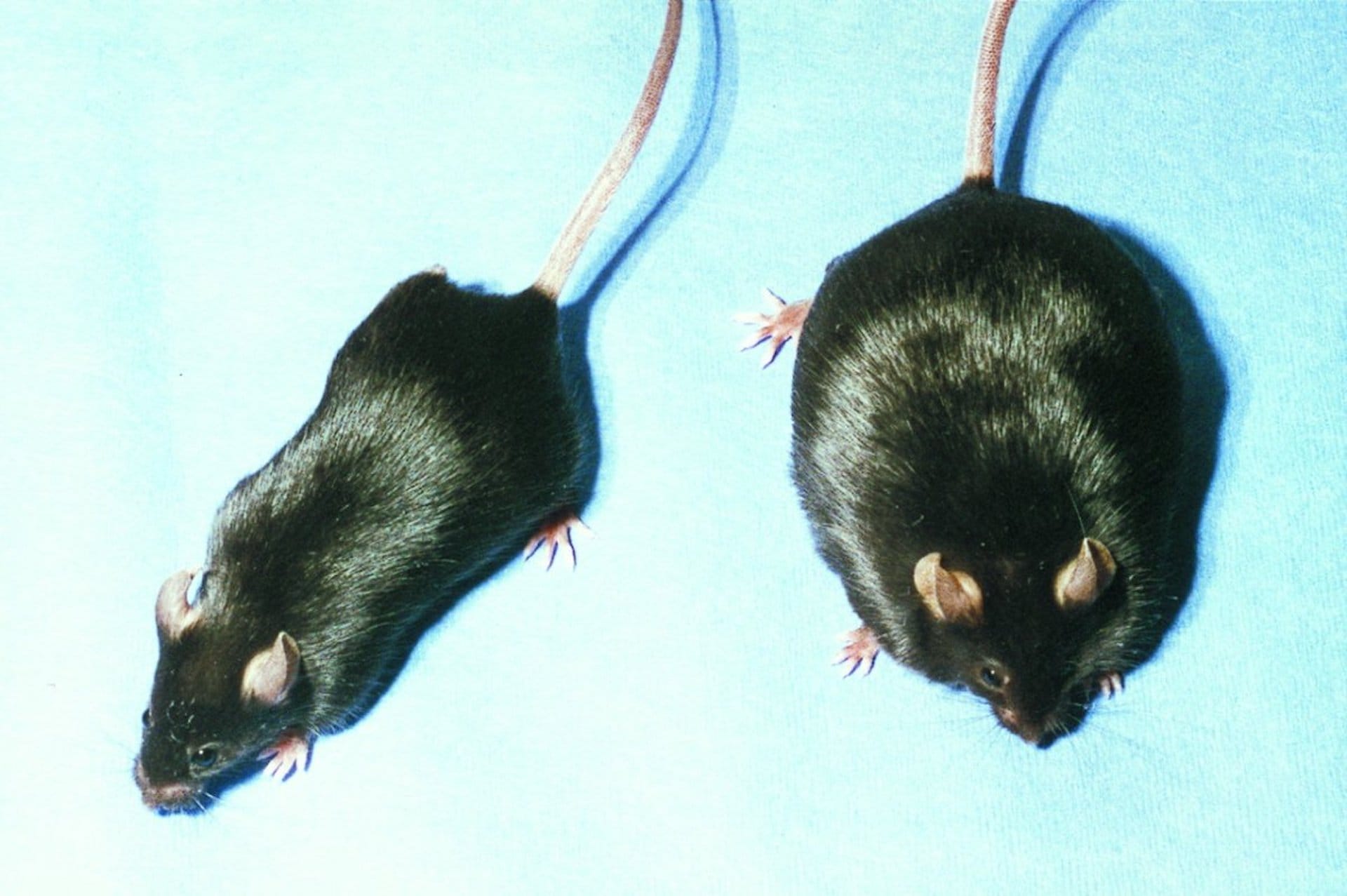
Clarence persisted until he managed to breed a robust, genetically identical colony that could withstand being inbred. The colony, called 6, had black fur and was bred from female mouse 57 and male mouse 52 – and so the colony was called C57Black6, or C57BL/6 for short.
The C57Black6 mouse is still used in research today – and has led to countless scientific insights into biology and the way our bodies work.

Since Clarence’s breeding experiments, countless scientific experiments have flowed from the C57Black6 mouse colony, in thousands of laboratories around the world. It’s led to at least 17 Nobel prizes, vital new cancer treatments and profound scientific insights into biology, like the way the immune system works and how we inherit traits from our parents.
It’s ideal for genetics and medical research, due to its ability to stay as a reproducing family of genetically robust clones.
'Understanding the role of any single gene is hugely complex. That’s because any characteristic or disease is normally the result of many different genes and the environment,' explains James Bussell, who headed up the Wellcome Sanger Institute’s Research Support Facility until 2018. 'However, if you can control the genetics of the animal and the environment that the animal lives in, and then change one single gene – then you can begin to say, “ah, that gene is involved in this process” '.
That might sound complex – but it’s possible to take two groups of C57Black6 and change just one gene in one group to study what happens. If the group with the affected gene develops a disease but the other group doesn’t, we can begin to explore the role the gene plays in the disease’s progression.
We now know that for nearly every human gene, there’s an equivalent gene in the mouse. “Because of its closeness to us in terms of the genetic makeup, we can begin to cross-correlate that to human disease states,” says James. This helps us to understand what the gene normally does and what effect mutations in that gene might have in humans.

For nearly every human gene, there’s an equivalent gene in the mouse.
Using the C57Black6 mouse to develop new cancer treatments
In the early 2000s, scientists at the Wellcome Sanger Institute discovered that half of all people with malignant melanoma, a type of skin cancer, had the same genetic mutation in their cancer cells.
The mutation was in a gene called BRAF (pronounced “Bee-Raff”). Following its discovery, scientists used C57Black6 mice to understand how the equivalent gene and mutation in mice causes cancer.
Collectively, this research led to the development of vital new cancer drugs, including vemurafenib, which was approved for use in people with malignant melanoma in late 2012. This has dramatically improved the outlook and outcomes of people with malignant melanoma – who previously had limited options available for treatment.
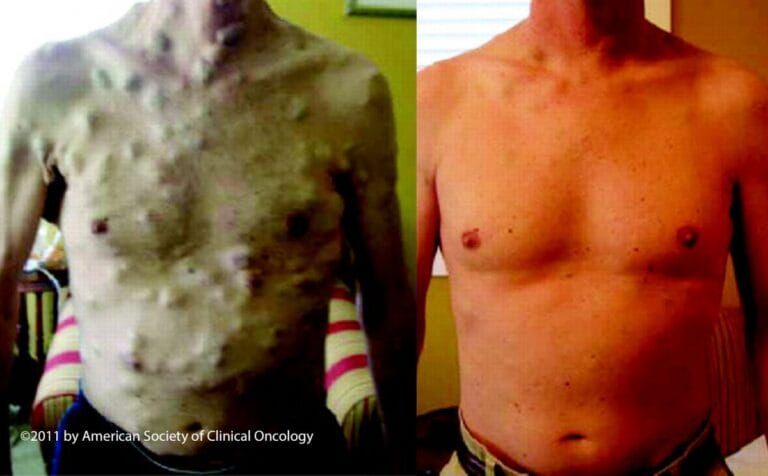
Image credit: American Society of Clinical Oncology
Other mice and their use in biomedical research
Just like C57Black6, other inbred mouse colonies are improving our understanding of human genetics and disease.
In 1949, Ann Ingalls, Margaret Dickie and George Snell were studying an inbred mouse called V/Le. They came across an unusual group of related mice with severe obesity and diabetes – and discovered that the obesity was due to an abnormally high desire for food.
After years of research, they found these mice all had the same mutation in a gene for a hormone called Leptin, which normally controls appetite and tells the body when the stomach is full. In these mice, the leptin gene no longer worked, so they were constantly hungry.
Since its discovery in mice, scientists have identified children with severe obesity who have similar mutations in the human leptin gene. Giving these children the Leptin hormone has proved to be an effective treatment that controls their appetite.
Thanks to the selective and inbreeding of mice, scientists now have a valuable tool to study the role of genetics in health and disease – leading to new, precise, effective treatments.