What is a telomere?
Image credit: Shutterstock
Telomeres are distinctive structures found at the ends of our chromosomes. They consist of the same short DNA sequence repeated over and over again.
Definition of telomeres
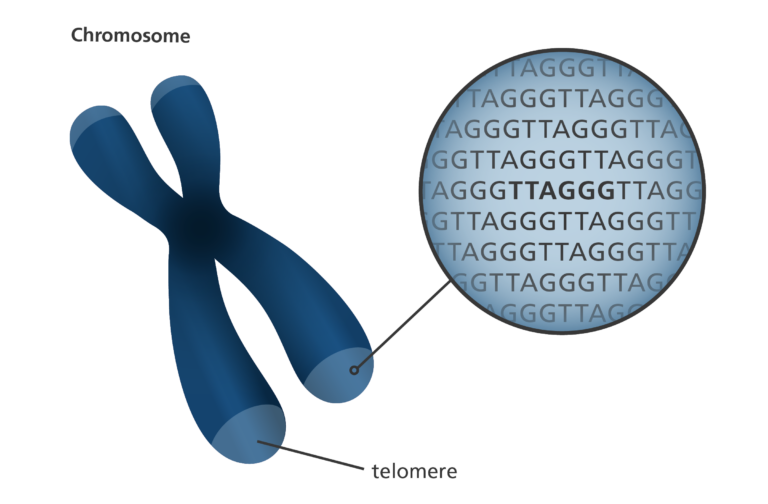
- Telomeres are sections of DNA found at the ends of each of our chromosomes.
- They consist of the same sequence of bases repeated over and over.
- In humans the telomere sequence is TTAGGG.
- This sequence is usually repeated about 3,000 times and can reach up to 15,000 base pairs in length.
What do telomeres do?
Telomeres serve three major purposes:
- They help to organise each of our 46 chromosomes in the nucleus (control centre) of our cells.
- They protect the ends of our chromosomes by forming a cap, much like the plastic tip on shoelaces. If the telomeres were not there, our chromosomes may end up sticking to other chromosomes.
- They allow the chromosome to be replicated properly during cell division:
- Every time a cell carries out DNA replication the chromosomes are shortened by about 25-200 bases (A, C, G, or T) per replication.
- However, because the ends are protected by telomeres, the only part of the chromosome that is lost is the telomere, and the DNA is left undamaged.
- Without telomeres, important DNA would be lost every time a cell divides (usually about 50 to 70 times).
- This would eventually lead to the loss of entire genes.
What happens to telomeres as we age?
- Each time a cell divides, 25-200 bases are lost from the ends of the telomeres on each chromosome.
- Two main factors contribute to telomere shortening during cell division:
- The ‘end replication problem’ during DNA replication: Accounts for the loss of about 20 base pairs per cell division.
- Oxidative stress: Accounts for the loss of between 50-100 base pairs per cell division. The amount of oxidative stress in the body is thought to be affected by lifestyle factors such as diet, smoking and stress.
- When the telomere becomes too short, the chromosome reaches a ‘critical length’ and can no longer be replicated.
- This ’critical length’ triggers the cell to die by a process called apoptosis, also known as programmed cell death.
How is telomere length maintained?
- Telomerase is an enzyme that adds the TTAGGG telomere sequence to the ends of chromosomes.
- Telomerase is only found in very low concentrations in our somatic cells. Because these cells do not regularly use telomerase they age leading to a reduction in normal function.
- The result of ageing cells is an ageing body.
- Telomerase is found in high levels in germline cells (egg and sperm) and stem cells. In these cells telomere length is maintained after DNA replication and the cells do not show signs of ageing.
- Telomerase is also found in high levels in cancer cells. This enables cancer cells to be immortal and continue replicating themselves. If telomerase activity was switched off in cancer cells, their telomeres would shorten until they reached a ‘critical length’. This would, prevent the cancer cells from dividing uncontrollably to form tumours.
- The action of telomerase allows cells to keep multiplying and avoid ageing.
Telomeres and research
- Research on telomeres and the role of telomerase could uncover valuable information to combat ageing and fight cancer.
- The medical relevance of telomeres is uncertain.
- Human cells cultured in the lab have been observed to stop dividing when telomerase is inactivated, because the length of telomeres is not maintained after cell division.
- The cells then enter a state of inactivity called senescence. However, once telomerase is reactivated, the cells are able to continue dividing.
- If telomerase can be used to help human cells live forever, it may also be possible to mass produce cells for transplantation. These cells could help to treat a range of conditions, from severe burns to diabetes.
Telomeres and ageing
- Mice models lacking the enzyme telomerase were found to show signs of premature ageing.
- However, it is not certain whether telomere shortening is responsible for ageing in humans or whether it is just a sign of ageing, like grey hair.
- There are several indications that telomere length is a good predictor of lifespan.
- Newborn babies tend to have telomeres ranging in length from around 8,000 to 13,000 base pairs. It has been observed that this number tends to decline by around 20-40 base pairs each year. So, by the time someone is 40 years old they could have lost up to 1,600 base pairs from their telomeres.
- However, looking at the bigger picture, the overall shortening of our telomeres is not significant, even in very old people.
- Cells that divide rapidly, such as germ cells and stem cells, are among the few cell types in our bodies containing active telomerase.
- This means that in these cells telomere length is maintained or even lengthened over time.
- However, there are a number of other factors that have an effect on the length of our telomeres that all need to be considered, such as smoking and obesity.
Telomeres and cancer
- Telomeres and telomerase present a number of potential targets for the design of new cancer therapies.
- Cancer cells contain active telomerase to enable them to become ‘immortal’ and continue dividing uncontrolled.
- Cancer is a disease characterised by the rapid and uncontrolled division of cells.
- Without telomerase activity, these cells would become inactive, stop dividing and eventually die.
- Drugs that inhibit telomerase activity, or kill telomerase-producing cells, may potentially stop and kill cancer cells in their tracks.
- However, blocking telomerase activity could affect cells where telomerase activity is important, such as sperm, eggs, platelets and immune cells.
- Disrupting telomerase in these cell types could affect fertility, wound healing and the ability to fight infections.
- However, telomerase activity in somatic cells is very low. These cells would therefore be largely unaffected by anti-telomerase therapy.
- Scientists hope this would result in fewer side effects for the patient, compared to current cancer therapies.
- Telomere biology is incredibly important in human cancer and scientists are working hard to understand the best way to exploit their knowledge of it to advance the treatment of cancer.