Malaria: the search for a vaccine
After decades of research, the world’s first safe, effective vaccine for malaria began being given to children at high-risk of the disease in 2019.
This is part 3 in ‘Using Genomics to Tackle Malaria’ series. You should first read part 1, exploring a deeper understanding of malaria and part 2, looking at how malaria is treated, before reading part 3.
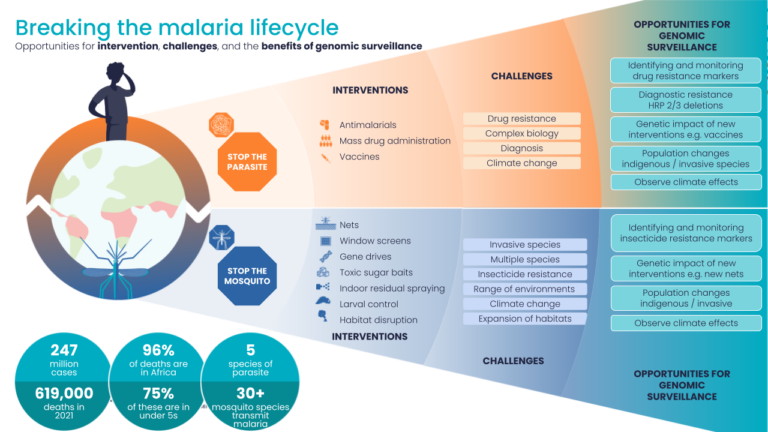
- Malaria is a life-threatening disease found in tropical and sub-tropical areas of Africa, South America and Asia. It’s caused by parasites that are carried from one human to another by mosquitoes.
- It’s taken decades of research, but in 2019, a vaccine against the parasites that cause malaria was finally approved. This was tested in pilot studies in 2019 and approved for wide-spread use in 2021.
- In the years since, the vaccine has slowly been rolled out across high-risk areas, helping to reduce the numbers of children dying from severe malaria. A second vaccine was approved in 2023.
Key terms
Genome
The complete set of genetic instructions required to build and maintain an organism.
Vaccine
A type of treatment that provides active acquired immunity against a specific pathogen.
Immunity
An organism’s ability to resist an infection or disease.

Between 2019 and 2023, more than 1.7 million children received at least one dose of the RTS,S malaria vaccine.
Looking for a vaccine to prevent malaria
We’ve known since 1880 that a group of parasites called Plasmodium are the cause of malaria, and since 1898 that mosquitoes are the vectors that spread them between people. Thanks to an increase in funding and awareness, the global community has introduced strategies to prevent the spread of infection, including distributing mosquito nets, spraying insecticides to kill mosquitoes and developing antimalarial drugs.
Between 2000 and 2015, this helped to reduce deaths from malaria by 47%. However, an ongoing challenge is that Plasmodium falciparum – the most serious of the species that can cause malaria – can become resistant to these treatments.
This prompted scientists around the world to collaborate to develop a vaccine against Plasmodium falciparum.
In 2019, the first ever vaccine for malaria – called RTS,S or Mosquirix – began to be given to children in Ghana, Kenya and Malawi as part of a pilot roll-out. In 2021, the World Health Organization recommended the vaccine should be used in more countries where children are at high-risk of developing malaria.

Vaccination is one of the most effective ways to control infectious diseases.
What is a vaccine?
Vaccination is one of the most effective ways to control infectious diseases. Unlike a drug, it uses the body’s natural defence system, the immune system, training it to recognise a particular pathogen like a bacteria or virus.
A vaccine usually contains a small amount of the pathogen, which the immune system learns to recognise as dangerous and to produce antibodies against it. Later, if the body is exposed to the real pathogen, the body already has an army of antibodies and knows how to launch a direct attack against it.
Vaccines have historically been very useful in the management of viruses (like smallpox and Sars-Cov-2, which causes Covid-19) and bacteria (like tetanus). However, until the malaria vaccine, no vaccine had ever been developed against a eukaryotic parasite like that which causes malaria.
Why was it so challenging to create a malaria vaccine?
For several reasons, it took decades for scientists to develop a safe, effective vaccine for malaria.
- Plasmodium is a complex organism
The Plasmodium species are protozoan parasites – meaning they’re eukaryotic cells, with similar complex cellular structures and life cycles to those found in the human body. Compared to the relatively basic structures of bacteria, this complexity made developing a Plasmodium vaccine much more of a challenge. - Its life cycle protects it from detection by the immune system
The parasite spends much of its life cycle inside red blood cells, hidden from the immune system. We cover this in more detail in part 2 of our malaria series. - It changes rapidly, helping to hide itself from the immune system
When Plasmodium enters the red blood cells, it puts proteins on the cell surface so that it can interact with other host cells. These proteins could be candidates for a vaccine – but the parasite has tactics to change these proteins too quickly for a vaccine to keep up. We cover this in more detail in part 2 of our malaria series.
Despite these challenges, scientists remained certain it would be possible to develop a vaccine for malaria. This is partly because people who are repeatedly infected with malaria and survive can develop a natural protection, known as natural immunity – and eventually the immune system learns how to protect against further infections.
Introducing the RTS,S malaria vaccine (aka Mosquirix)
How does the RTS,S malaria vaccine work?
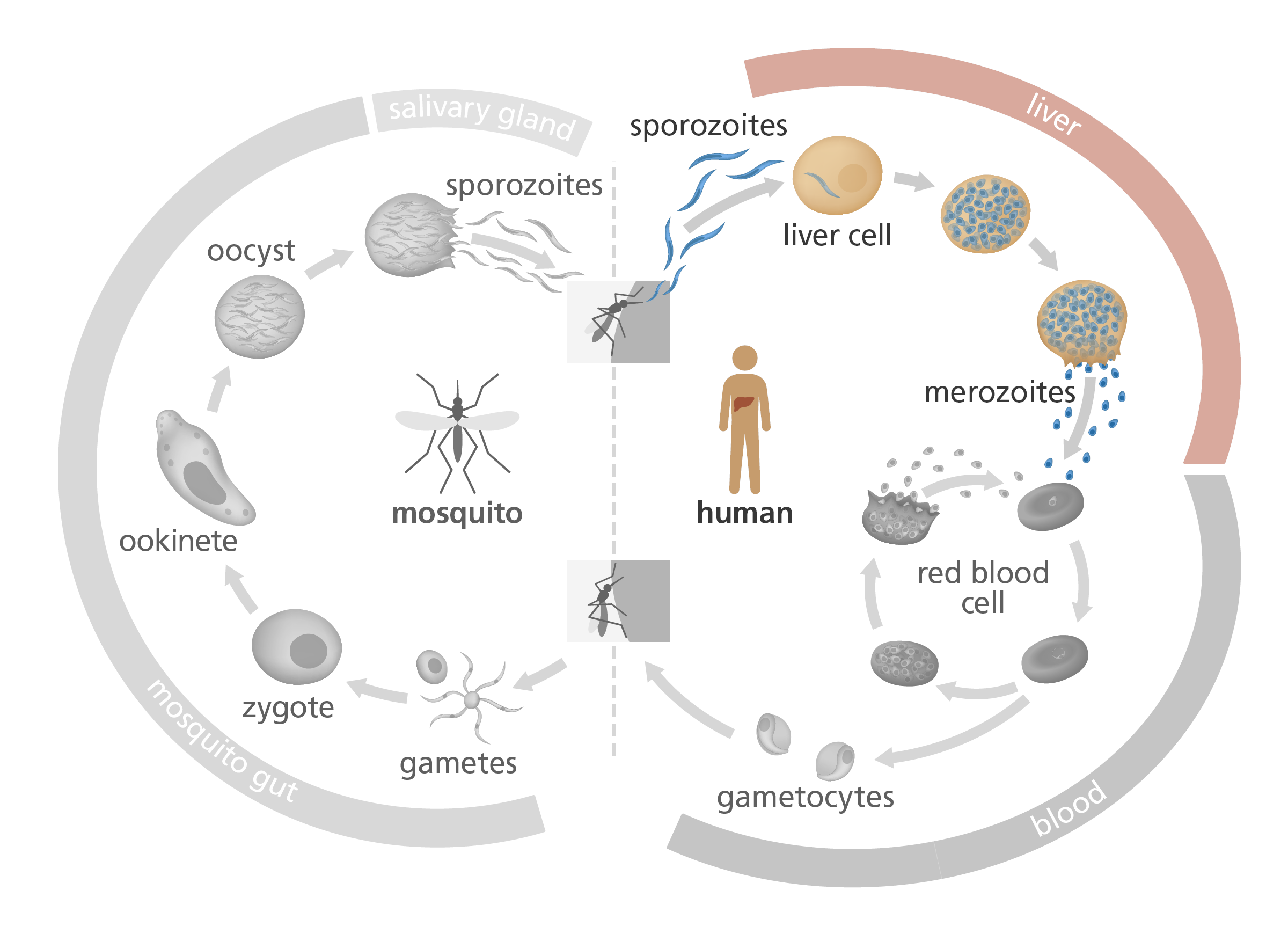
The complex Plasmodium life cycle takes place in mosquitoes and in humans. The RTS,S vaccine targets the sporozoite and liver stages of the Plasmodium life cycle, to prevent progression to the blood stage.
When Plasmodium first enters the bloodstream via a mosquito bite, it’s in the form of a sporozoite – a thin, immature parasite. It produces a protein called circumsporozoite protein (or CSP), which it wears on its surface and uses to find its way to the liver – a bit like a molecular satnav. Once in the liver, it matures and multiplies before invading red blood cells and causing malaria.
The RTS,S vaccine trains the immune system to recognise CSP. When the immune system finds CSP, it triggers an immune response, killing the parasite before it has the chance to reach the liver.
The long road to a vaccine
Developing the RTS,S malaria vaccine took more than 30 years and involved scientists from all over the world.
In 2015, the European Medicines Agency gave RTS,S (then marketed as Mosquirix) the green light for safety and effectiveness. A pilot rollout began in 2019 across Ghana, Kenya and Malawi, before the World Health Organization recommended its use in 2021 in areas of greatest need, including Benin, Burkina Faso, Burundi, Cameroon, the Democratic Republic of the Congo, Liberia, Niger, Sierra Leone and Uganda.
Between the roll-out in 2019 and 2023, more than 1.7 million children received at least one dose of the RTS,S vaccine. At least 28 countries in regions of Africa with high risk of malaria expressed interest in deploying it as part of their national malaria control strategies.
How effective is the RTS,S malaria vaccine?
Many studies have proven RTS,S is safe and effective against malaria caused by the Plasmodium falciparum parasite.
Since its pilot rollouts in 2019, the vaccine has dramatically reduced the number of cases of severe malaria in Ghana, Kenya and Malawi, and helped to reduce the number of children dying from the disease.
However, while the vaccine is effective in the short-term, it’s unclear how long its protection will last. The best level of protection is when children aged 5-17 months receive three doses of the vaccine a month apart, followed by a booster dose at 20 months. So far, we know this schedule can protect patients for up to five years – but it’s likely that other measures will be needed to help prevent malaria in the long-term.
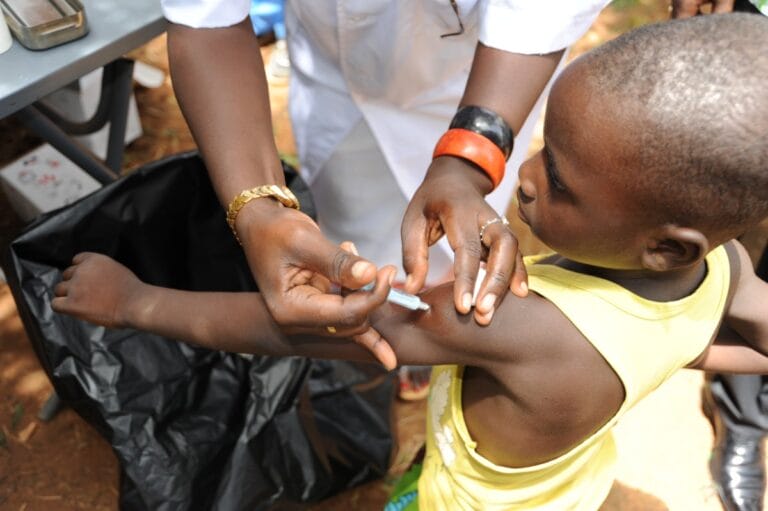

Malaria kills, but there is now a vaccine that we know works and can be implemented. If all the doses are taken, we know it prevents severe forms of malaria.
Dr Don Mathanga, University of Malawi’s College of Medicine, speaking to the World Health Organisation in 2023.
A second vaccine for malaria
In October 2023, a second vaccine for malaria was approved. The new vaccine, called R21/Matrix-M, works in a similar way to RTS,S by targeting the sporozoite stage of the parasite’s life cycle and has been shown to be just as safe and effective.

As a malaria researcher, I used to dream of the day we would have a safe and effective vaccine against malaria. Now we have two. This second vaccine is a vital additional tool to protect more children faster, and to bring us closer to our vision of a malaria-free future.
Dr Tedros Adhanom Ghebreyesus, WHO Director-General, speaking to the World Health Organisation in 2023.
Looking beyond the current malaria vaccines: what’s next for malaria prevention?
Scientists are currently looking for additional strategies that could be used in combination with or instead of the vaccines to boost our ability to prevent malaria.
A combined vaccine approach
A collaboration between scientists at the Kenya Medical Research Institute and the Wellcome Sanger Institute in the UK found that children are more protected against malaria when they have antibodies against lots of different Plasmodium proteins, not just CSP. A vaccine that has multiple targets, rather than a single ‘magic bullet’, might be a more effective preventative approach.
A vaccine to block transmission
Transmission-blocking vaccines could help prevent the transmission of malaria by preventing mosquitoes from becoming infected with Plasmodium when it feeds on an infected person. This works by injecting infected people with a vaccine that induces antibodies in the mosquito that prevent the parasite from maturing inside the mosquito’s gut.
Although this approach wouldn’t directly prevent infection in the vaccinated person, it could help to reduce transmission and potentially protect wider communities against future outbreaks of the disease.
Super vaccines that target multiple stages of the Plasmodium lifecycle
Although it’s the blood stage of the Plasmodium life cycle that actively causes clinical disease, we probably need to target multiple life stages to develop a really effective malaria vaccine. That means combining the best transmission-blocking vaccine with the best liver stage vaccine and the best blood stage vaccine, to produce a ‘super’ vaccine to prevent malaria.
However, an ideal vaccine should be relatively cheap and easy to administer. Developing a super vaccine like this would likely be an expensive, complicated process – so a balance must be found between effectiveness and cost.
Overcoming resistance
Just like a pathogen can become resistant to drugs, it can become resistant to a vaccine. Scientists need to consider the evolutionary dynamics of Plasmodium when working on a long-term preventative approach.
Realistically, the fight against malaria is going to be a long one
Realistically, the fight against malaria – whether with insecticides, drugs or vaccines – is going to be a long one, with many approaches needed at the same time.
In part 2 of this series, we introduce the Red Queen hypothesis – an evolutionary theory that proposes organisms must constantly adapt and evolve in order to survive against their ever-evolving opposition. Sometimes, this can feel like doing all the running you can do, simply to stay in the same place: while we develop the next generation of antimalarial drugs or vaccines, the malaria parasite is already planning its next move.
But we are making progress. In 2021, 35 countries reported fewer than 1000 cases of malaria, up from 33 countries in 2020 and just 13 countries in 2000. And between 2015 and 2021, nine countries were declared malaria-free, with zero cases of malaria, including the Maldives, Sri Lanka, Uzbekistan and China.
By combining all available preventative strategies and treatments, the World Health Organization believes we will be able to reduce malaria cases by at least 90% before the year 2030 – an ambitious goal, but an achievable one.